Designer and manufacturer of processing equipment Anderson Engineering’s AndPure Magnetic Mixer, fabricated entirely from stainless steel and used to mix liquid products, is particularly popular in the pharmaceuticals industry, owing to its ultrahygienic properties, says Anderson Engineering MD Hans Coertze.
The mixer, a Southern Africa Stainless Steel Development Association 2016 Columbus Stainless Awards finalist, can be used in any vessel or manufacturing environment. Since its inception in 2013, this South African-developed mixer has undergone a number of upgrades and improvements, resulting in the introduction of a larger model developed and introduced this year.
“The design and engineering of the Anderson Magnetic Mixer catered to the pharmaceuticals industry’s need to find a solution for the ultrahygienic mixing of certain products. The sole purpose of the mixer is to ensure an ultra- hygienic product, a result that stainless steel delivers,” Coertze denotes.
He describes the mixer as the most advanced mixer for dissolvable products that require the highest level of hygiene.
The mixer can be used to mix liquid products in the food and beverage and chemicals industries.
Coertze points out that, compared with the Andpure Magnetic Mixer, traditional mixing methods use shaft-driven impellers or mixing blades that protrude out of the vessel and are normally supported by an outside bearing from the point of entry to the vessel using a mechanical seal.
Mechanical seals consist of numerous components, which include pressure faces, stainless steel backing flanges and springs, which, together with locking screws, provide sealing in the mixer.
Coertze says achieving an ultrahygienic design with mechanical seals is “extremely difficult”, which invariably leads to compromise on the hygiene aspect of the process.
The failure of mechanical seals is a common occurrence in mixers, resulting in either product loss, batch contamination or both. Coertze notes that product loss and batch contamination are unacceptable in the pharmaceuticals industry. It was these issues that Anderson Engineering identified as challenges their clients encountered that needed to be overcome.
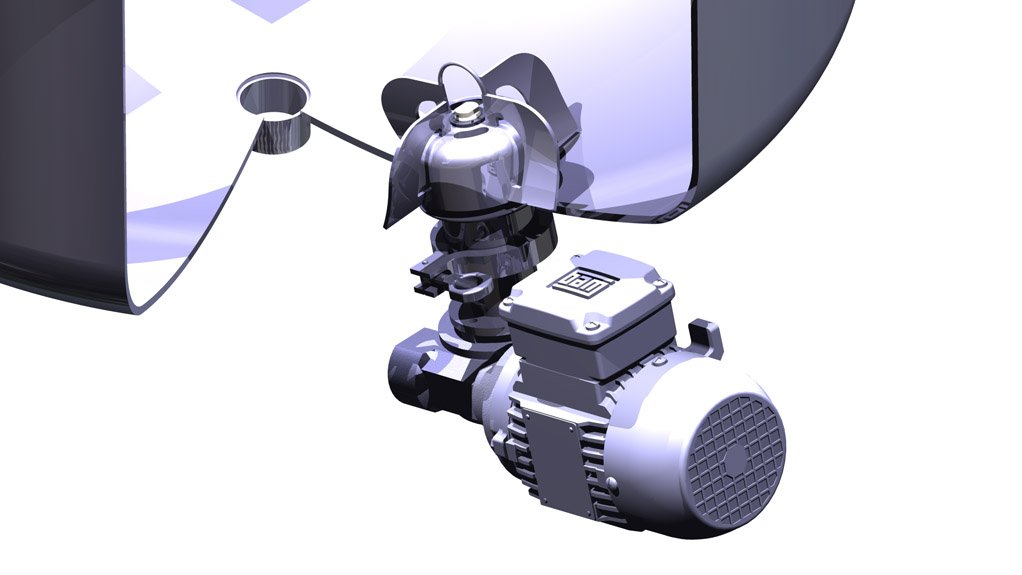
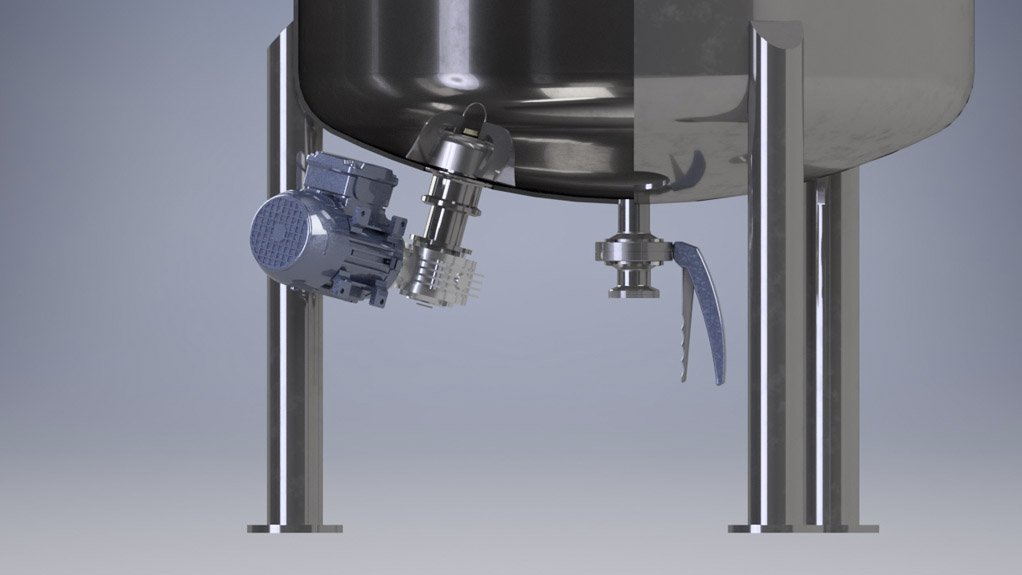
Meanwhile, Coertze explains that the magnetic driver system uses a stainless steel fabricated and fully welded casing through which a mixing element is driven on the inside of the vessel, using magnetic fields from the outside rotor.
The magnetic driver system does not have any crevices or potential leakage points, which, according to Coertze, is ideal for the establishment of a completely sterile and ultrahygienic mixer.
“The mixer design has no seals, which ensures that there are no areas where products can get trapped. It improves operational efficiencies, owing to less maintenance of the system, compared with the high maintenance of mechanical seal systems,” he explains.
The AndPure Magnetic Mixer system enables the manufacture of products such as intravenous medications and other products that require high standards of hygiene.
“A combination of watching international trends and solutions, combined with the demands of our local pharmaceuticals industry, as well as our expertise in mixing solutions, led to the conceptualisation of this innovative solution,” Coertze says.
Further, while the mixer is small, measuring less than 15 cm, it can mix up to 300 ℓ of liquids in tanks, with power consumption of 0.37 kW; the larger model, measuring 23 cm, can be fitted to tanks and can mix up to 1 000 ℓ of liquids.
The AndPure Magnetic Mixer was used for a bulk solutions preparation by pharmaceuticals company Aspen Pharmaceuticals, in Port Elizabeth, Coertze says. The mixer has also been used in KwaZulu-Natal, in a 300 ℓ process vessel for therapeutic proteins and diagnostic products manufacturer and supplier National Bioproducts Institute.
Meanwhile, Coertze says the mixer incorporates a three- to five-minute installation process which, he enthuses, is ideal for audits, checks, and quick assembly and disassembly. However, he notes that the initial fabrication stage takes several hours to fabricate into the vessel in which it is to be used.
“The mixer can be disassembled in less than three minutes and the mixing element can be removed, while the motor geared drive is detachable from the vessel, allowing for the mixing element and vessel to be fully sterilised using autoclave technology. The components might be reassembled after sterilisation,” he concludes.
Edited by: Zandile Mavuso
Creamer Media Senior Deputy Editor: Features
EMAIL THIS ARTICLE SAVE THIS ARTICLE
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here