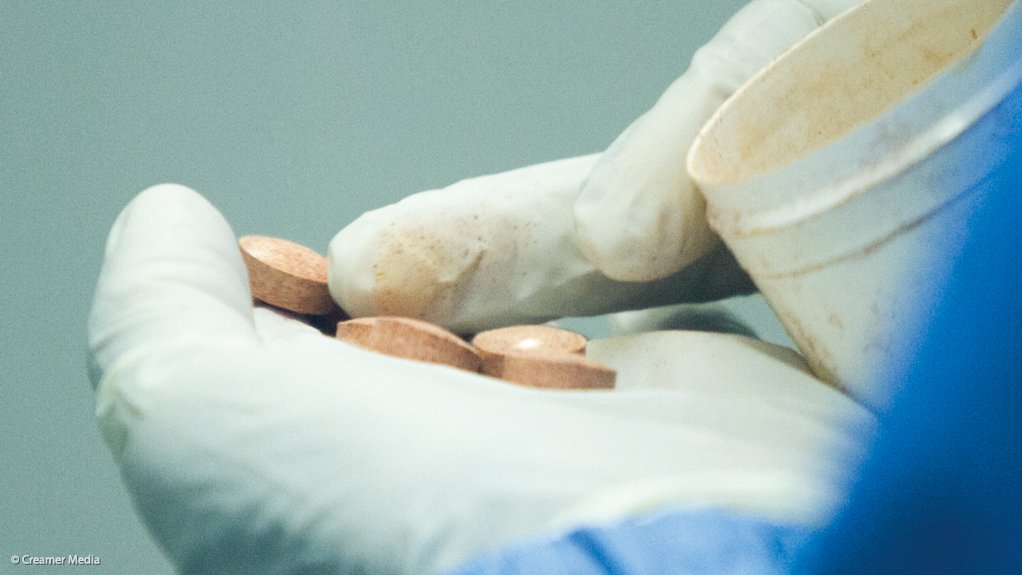
During his State of the Nation Address, President Jacob Zuma announced that a State-owned pharmaceutical company called Ketlaphela had been established.
He noted that “the company will participate in the supply of antiretroviral drugs to the Department of Health from the 2016/17 financial year”.
The Department of Science and Technology (DST) says that “it is envisaged that the first Ketlaphela branded tablets will be available at the beginning of 2017”.
Ketlaphela, which means “I will survive” in Sesotho, was actually started by government as long ago as 2007.
Originally it was set up as a joint venture between the Industrial Development Corporation, a Swiss pharmaceutical company called Lonza and Pelchem, a manufacturer of fluorochemicals which is owned by the South African government, for the purpose of locally producing the active pharmaceutical ingredients (APIs) for antiretroviral drugs (ARVs) used to treat HIV/AIDS.
In 2013, Lonza pulled out of the joint venture, leading some commentators to question the financial viability of the project.
According to the DST, Ketlaphela SOC Limited is now a registered company and a 100% subsidiary of Pelchem that is “at an advanced stage of negotiations with the Geneva-based Medicines Patent Pool which would provide not only the licences to manufacture the latest patent-protected ARVs but access to the know-how / technologies”.
The Medicines Patent Pool is a public health organisation that partners with governments, industry, civil society and other stakeholders to increase access to important medications in low- and middle-income countries by encouraging the production of generic drugs and the development of new formulations through patent pooling.
With regards to Ketlaphela’s financial feasibility, the DST emphasises that the company “will ensure that its business model is sustainable and not a burden on the South African taxpayers”, insisting that “the start-up funding required by Ketlaphela is modest”.
As the world’s biggest consumer of ARVs, South Africa spends billions of rands purchasing the drugs on the international market every year.
Manufacturing them within the country potentially means lower prices, improved access and more secure supplies, along with job creation and the development of an indigenous knowledge and skill base in the field. But until now, progress has been slow.
The DST describes Ketlaphela as aiming to bridge “the gap between the research and development capabilities in South Africa, by adding chemical manufacturing (API), integrated with secondary pharmaceutical manufacturing (tablet formulation), targeting the burden of diseases, initially for South Africa and subsequently expanding into SADC and Southern Africa.The goal of Ketlaphela is to establish itself as a supplier to government whilst transferring modern novel technologies and introducing new generation drugs for local manufacturing”.
Currently, the company does not have its own manufacturing facilities. It intends to establish itself as a supplier, outsourcing the formulation of medications to South African companies with spare capacity until it is in a position to set up in-house manufacturing operations with state-of-the-art technologies.
A Ketlaphela facility to manufacture semi-commercial quantities (up to a few tonnes per year) of APIs in Pretoria is under consideration, says the DST, adding that the company ”will not only target ARVs but also drugs where there is a sustained security of supply risk, such as some antibiotics and oncology medicines”.
Edited by: News24Wire
EMAIL THIS ARTICLE SAVE THIS ARTICLE
To subscribe email subscriptions@creamermedia.co.za or click here
To advertise email advertising@creamermedia.co.za or click here